- GST No. 27AACPV0352J1ZM
1.00 / Box
| Business Type | Manufacturer, Exporter, Supplier, Retailer, Wholesaler |
| Country of Origin | India |
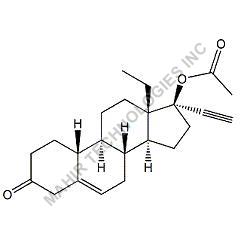
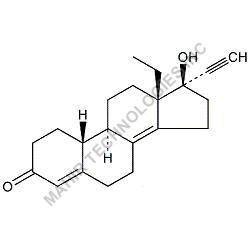
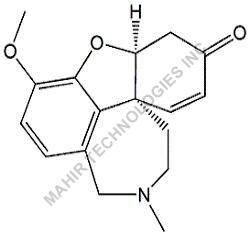
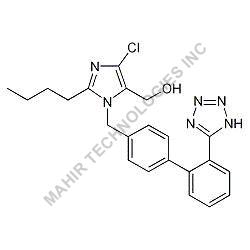
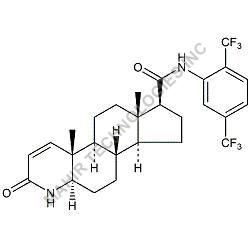
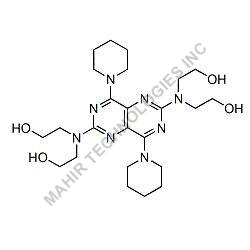
| Molar mass | 312.446 g/mol |
| Formula | C21H28O2 |
| Click to view more | |
Preferred Buyer From
| Location | Anywhere in India |
Product Details
Elimination half-life
24–32 hours
Metabolism
Liver (reduction, hydroxylation, conjugation)
Grade
Pharma
Mol. Wt.
312.45
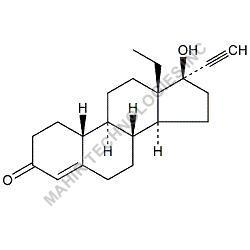
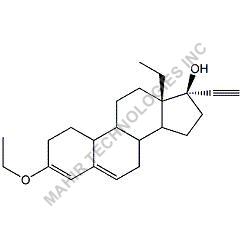
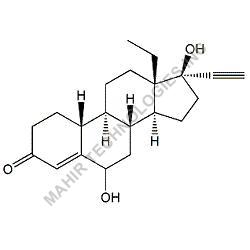
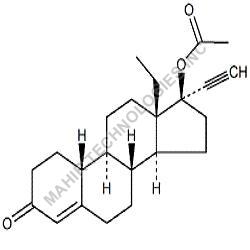
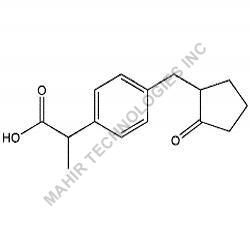
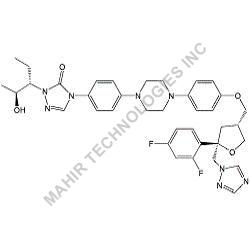
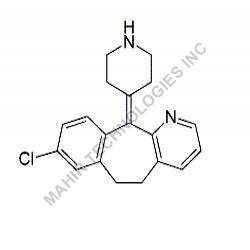
We synthesis Levonogestral Impurities as per the Pharmacautical methods as mentioned in USP and are readily available
Looking for "Levonorgestrel Impurity" ?
Box
Explore More Products
Our Blogs